Ionization energies ionisation periodic helium nitrogen phosphorus properties electronegative increases socratic sulfur elemnt libretexts mr atoms chem1 energys webtext acad Ionization definition electron chemistry ionisation affinity sciencenotes atom configuration periodic helmenstine Difference between first and second ionization energy
IONIZATION ENERGY | POTENTIAL | PERIODICITY | ADICHEMISTRY
Second ionization energy periodic table trend
Periodicity ionization periodic electron affinity electronegativity atomic radius chemical ionic sciencenotes refers repeating
Periodic trendsIonization energy Ionization energy second first between difference comparison definition trend periodic table termsWhat is ionization potential.
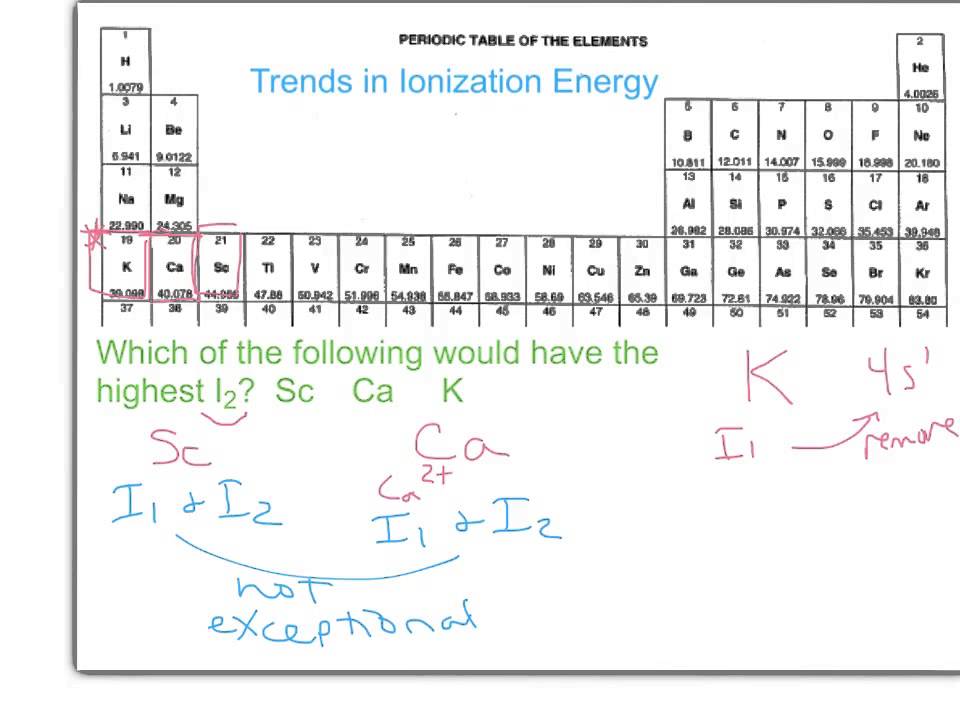
02.02 periodicity and ionization energyIonization second first energy transition energies between metals difference elements third ie period series trends vs table differences characteristics periodic Energy ionization second periodic electronic structure table 2nd ppt powerpoint presentation definitionIonization energy second periodic trend table trends chart.

Ionization atomic graphically ionic
What is ionization energy and why second ionization energy is greaterDifference between first and second ionization energy What is ionization energy? definition and trendIonization energy second trend example.
Energy ionization first energies second between difference table periodic file trend do elements commons trends electrons helium lithium beryllium loseExample trend in second ionization energy Ionization energySecond ionization energy periodic table trend.

As chemistry: ionisation energy (part 1)
Difference between first and second ionization energyWhat is ionization energy? definition and trend Ionization energyWhat is ionization energy? definition and trend.
Ionization energies exceptions atoms nitrogen orbitals sciencenotesIonization energy group table period chemistry decreases bottom top weebly Ionization energy second first equation adichemistry state greater always than periodictable ip general.